The average atomic mass of zirconium is 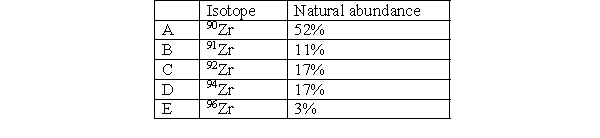
A) less than 90 because the atomic mass only depends on the number of protons in the atom.
B) 90 because 90Zn has the highest natural abundance.
C) greater than 90 but less than 96 because the atomic mass takes into account the abundance of all naturally occurring isotopes.
D) 96 because the atomic mass is the mass of the highest naturally occurring isotope.
E) greater than 96 because the atomic mass is the sum of masses of the naturally occurring isotopes.
Correct Answer:
Verified
Q8: According to the periodic table, the atomic
Q18: The most common nutritional deficiency in the
Q19: According to the current model of the
Q26: According to the periodic table, which of
Q27: What is the identity of element X?
Q28: An element is a solid at room
Q45: According to the periodic table, which element
Q60: _ make up the majority of compounds
Q90: _ determine the physical and chemical characteristics
Q92: According to the periodic table, which element
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents