Ascorbic acid, or vitamin C, is a water soluble vitamin. Which of the following interactions is primarily responsible for ascorbic acid's water solubility? 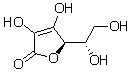
A) dispersion forces
B) dipole-dipole forces
C) hydrogen bonding forces
D) ionic bonding
E) covalent bonding
Correct Answer:
Verified
Q11: Select the choice that correctly classifies vitamins
Q12: What does [O] represent in the following
Q13: Is the alkene oxidized or reduced during
Q15: Cellular respiration is an example of a(n)
Q17: Which of the following is a reason
Q19: Which statement best describes why vitamin A
Q21: The following reaction is the hydrogenation of
Q48: In an oxidation-reduction reaction, the species that
Q54: In an oxidation-reduction reaction, the species that
Q61: An oxidation-reduction reaction is the transfer of
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents