Which statement best describes the following reaction? 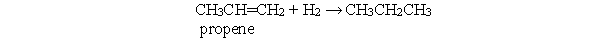
A) In this reaction, both propene and hydrogen are reduced.
B) In this reaction, both propene and hydrogen are oxidized.
C) In this reaction, propene is reduced and hydrogen is oxidized.
D) In this reaction, propene is oxidized and hydrogen is reduced.
E) This is not an oxidation-reduction reaction.
Correct Answer:
Verified
Q31: Foods that contain the product of the
Q34: Sorbitol is a sweetener often used in
Q34: Which functional group can be oxidized but
Q36: What is the product when this compound
Q37: Sorbitol is a sweetener often used in
Q38: Is the ketone oxidized or reduced during
Q38: Which classification of alcohols can undergo oxidation
Q39: Carbohydrates are molecules that are composed of
Q54: What is the purpose of NAD+ in
Q64: When unsaturated fatty acids are hydrogenated, an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents