Is the aldehyde oxidized or reduced during the following reaction, and how can you tell? 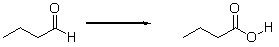
A) Neither. This is not an oxidation-reduction reaction.
B) The aldehyde is neither oxidized nor reduced because the number of hydrogens does not change.
C) The aldehyde is oxidized because it loses two hydrogens.
D) The aldehyde is reduced because it loses two hydrogens.
E) The aldehyde is oxidized because it gains an oxygen.
Correct Answer:
Verified
Q17: Which of the following is a reason
Q19: Which statement best describes why vitamin A
Q21: The following reaction is the hydrogenation of
Q23: Which of the following statements describes coenzymes?
A)
Q24: The reaction of fatty acid A is
Q27: Which vitamin is NAD+/NADH produced from in
Q33: Which food is NOT high in niacin?
A)
Q48: In an oxidation-reduction reaction, the species that
Q54: In an oxidation-reduction reaction, the species that
Q76: Which classification of alcohols cannot be oxidized,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents