Use the following data to calculate the standard heat (enthalpy) of formation, Hf, of manganese(IV) oxide, MnO2 (s) . 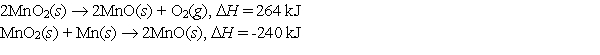
A) -504 kJ
B) -372 kJ
C) -24 kJ
D) 24 kJ
E) 504 kJ
Correct Answer:
Verified
Q43: Ethanol, C2H5OH, is being promoted as
Q43: Starting from equations relating pressure to
Q44: Use Hess's Law to calculate the
Q45: The highly exothermic thermite reaction, in which
Q46: Calcium hydroxide, which reacts with carbon dioxide
Q47: Which one of the following equations
Q49: Calculate the
Q50: Nitric acid, which is among the
Q51: Which one of the following statements
Q53: An important step in the synthesis
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents