Predict the products by completing a balanced equation for the following decomposition reaction. 
A) CaCl2(l) 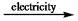 Ca(l) + 2Cl¯(l)
Ca(l) + 2Cl¯(l)
B) CaCl2(l) 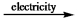 Ca2+(l) + Cl2(g)
Ca2+(l) + Cl2(g)
C) CaCl2(l) 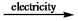 Ca2+(l) + 2Cl¯(l)
Ca2+(l) + 2Cl¯(l)
D) CaCl2(l)  Ca(l) + Cl2(g)
Ca(l) + Cl2(g)
E) CaCl2(l) 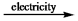 CaCl(l) + Cl¯(l)
CaCl(l) + Cl¯(l)
Correct Answer:
Verified
Q5: The combustion of an element is always
Q12: All combustion reactions are classified as combination
Q14: A combination reaction may also be a
Q15: Chemical reactions generally reach equilibrium because one
Q16: In a redox reaction, the oxidizing agent
Q17: A particular reaction may be both a
Q18: Covalent compounds, dissolved in water, never produce
Q19: Oxidation is associated with an increase in
Q91: An aqueous solution of lead nitrate, Pb(NO3)2,
Q95: A) Explain or define what is meant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents