Potassium is produced industrially by the reaction Na(l) + K+(l) 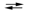 Na+(l) + K(g). The reaction is carried out at 850 C, but the equilibrium Kc constant strongly favors the reactants. Explain how the conditions are manipulated to ensure a good yield of potassium despite the small value of Kc.
Na+(l) + K(g). The reaction is carried out at 850 C, but the equilibrium Kc constant strongly favors the reactants. Explain how the conditions are manipulated to ensure a good yield of potassium despite the small value of Kc.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: The bond energy in the hydrogen molecule
Q7: The purpose of anodizing aluminum is to
Q11: In the industrial electrolysis of aqueous NaCl
Q20: The industrial production of ammonia from nitrogen
Q43: Compared to the asbestos diaphragm-cell used in
Q45: Lightning, fires and other atmospheric processes result
Q46: What gas is produced at the anode
Q50: .
Q50: In the commercial production of aluminum
Q53: Aluminum and magnesium form are light metals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents