Examine the following half-reactions and select the weakest oxidizing agent among the species listed. 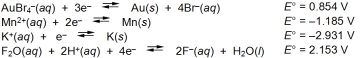
A) AuBr4¯(aq)
B) Mn2+(aq)
C) K+(aq)
D) F2O(aq)
E) H+(aq)
Correct Answer:
Verified
Q17: Consider the reaction: CuO(s) + H2(g)
Q18: Which one of the following pairs of
Q19: When the following redox equation is balanced
Q20: When the following redox equation is balanced
Q21: What is the E
Q24: A cell can be prepared from
Q25: Examine the following half-reactions and select the
Q26: The voltaic cell made up of
Q27: Calculate E
Q60: A voltaic cell has a standard cell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents