Calculate E cell and indicate whether the overall reaction shown is spontaneous or nonspontaneous. 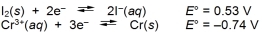 Overall reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I¯(aq)
Overall reaction: 2Cr(s) + 3I2(s) 2Cr3+(aq) + (aq) + 6I¯(aq)
A) E cell = -1.27 V, spontaneous
B) E cell = -1.27 V, nonspontaneous
C) E cell = 1.27 V, spontaneous
D) E cell = 1.27 V, nonspontaneous
E) E cell = 1.54 V, spontaneous
Correct Answer:
Verified
Q25: The line notation, Pt | H2(g) |
Q33: The redox reaction of peroxydisulfate with
Q35: Calculate E
Q35: When metal A is placed in
Q36: Examine the following half-reactions and select the
Q37: Calculate E
Q40: Examine the following half-reactions and select the
Q42: Calculate
Q43: What is the value of the
Q50: A battery is considered "dead" when
A)Q <
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents