The following half-reactions occur in the mercury battery used in calculators. If E 1U1B1cell = 1.357 V, calculate the equilibrium constant for the cell reaction at 25 C. (Assume the stoichiometric coefficients in the cell reaction are all equal to 1.) HgO(s) + H2O(l) + 2e¯ 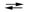 Hg(l) + 2OH¯(aq)
Hg(l) + 2OH¯(aq)
ZnO(s) + H2O(l) + 2e¯ 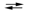 Zn(s) + 2OH¯(aq)
Zn(s) + 2OH¯(aq)
A) 9.4 * 1022
B) 7.5 *1045
C) 6.4 * 1063
D) 7.8 * 1091
E) > 9.9* 1099
Correct Answer:
Verified
Q55: Calculate the potential of a voltaic
Q56: A concentration cell consists of two
Q57: A voltaic cell consists of a
Q58: A voltaic cell consists of a
Q59: Consider the non-aqueous cell reaction
Q61: What product forms at the anode during
Q62: In the electrolysis of water, how
Q65: A current of 250. A flows
Q72: Chromium metal is electroplated from acidic aqueous
Q77: In the electrolysis of aqueous sodium sulfate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents