Consider the reaction of iodine with manganese dioxide:
3I2(s) + 2MnO2(s) + 8OH¯(aq) 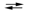 6I¯(aq) + 2MnO4¯(aq) + 4H2O(l)
6I¯(aq) + 2MnO4¯(aq) + 4H2O(l)
The equilibrium constant for the overall reaction is 8.30 *10¯7. Calculate E cell for the reaction at 25 C.
A) -0.36 V
B) -0.18 V
C) -0.12 V
D) -0.060 V
E) None of these choices is correct.
Correct Answer:
Verified
Q48: A voltaic cell consists of a
Q49: Calculate
Q50: Consider the reaction of iodine with
Q51: What is the value of the
Q52: Calculate E
Q54: The value of E
Q55: Calculate the potential of a voltaic
Q56: A concentration cell consists of two
Q57: A voltaic cell consists of a
Q58: A voltaic cell consists of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents