A concentration cell is based on the aqueous reaction 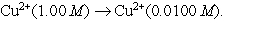 The cell consists of copper electrodes dipping into solutions of Cu2+ ions. The anions present are sulfate ions. Draw a neat diagram to represent this cell, showing and labeling all necessary components including: anode, cathode, electron flow, cation flow and anion flow.
The cell consists of copper electrodes dipping into solutions of Cu2+ ions. The anions present are sulfate ions. Draw a neat diagram to represent this cell, showing and labeling all necessary components including: anode, cathode, electron flow, cation flow and anion flow.
Correct Answer:
Verified
Q2: Electrolytic cells utilize electrical energy to drive
Q10: A salt bridge provides a path for
Q20: Electrons are produced at the cathode of
Q65: Predict the products of the cell reaction
Q71: In the electrolysis of aqueous potassium nitrate
Q72: Which of the following elements can be
Q75: What product forms at the cathode during
Q77: Two cells are connected in series,
Q81: A) Write a balanced equation to represent
Q84: A solution is prepared by dissolving 32.0
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents