Nitric oxide reacts with chlorine to form NOCl. The data refer to 298 K. 
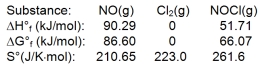 What is the value of G for this reaction at 550 K?
What is the value of G for this reaction at 550 K?
A) -143.76 kJ
B) -78.78 kJ
C) -22.24 kJ
D) -10.56 kJ
E) 66600 kJ
Correct Answer:
Verified
Q54: Given: H2O(l)
Q55: Elemental boron can be formed by
Q56: A certain process has
Q57: In order for a process to be
Q58: Calculate
Q59: In order for a process to
Q61: The formation constant for the reaction
Q62: In tables of thermodynamic data provided
Q63: Given: C2H2(g)
Q64: The water-gas shift reaction plays an important
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents