Use the thermodynamic data at 298 K below to determine the Ksp for barium carbonate, BaCO3 at this temperature. 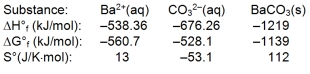
A) 5.86
B) 6.30 * 108
C) 1.59 * 10¯9
D) 5.47 * 10¯21
E) 2.18 * 10¯27
Correct Answer:
Verified
Q10: The higher the pressure of a gas
Q16: The entropy of one mole of oxygen
Q63: Given: C2H2(g)
Q64: The water-gas shift reaction plays an important
Q65: A reaction has a positive value
Q67: A reaction has
Q69: Iron(III) oxide can be reduced by carbon
Q69: In the expression, S = k ln
Q70: The reaction of methane with water to
Q79: State the second and third laws of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents