Given: 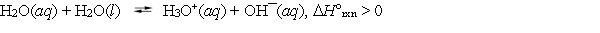 When the temperature of a sample of pure water is raised above 25 C,
When the temperature of a sample of pure water is raised above 25 C,
A) the hydronium ion concentration will be greater than the hydroxide ion concentration.
B) the hydronium ion concentration will be less than the hydroxide ion concentration.
C) the value of Kw will increase.
D) the hydronium ion concentration could change to 1.0 *10¯10 M.
E) the hydroxide ion concentration could change to 1.0 *10¯10 M.
Correct Answer:
Verified
Q1: Which, if any, of the following acids
Q24: Which of the following is the strongest
Q26: The substance NaNO3 is considered
A)a weak Arrhenius
Q27: The substance Ba(OH)2 is considered
A)a weak Arrhenius
Q28: Select the strongest acid from the following
Q30: Which of the following is the strongest
Q34: Which of the following pairs has the
Q36: The substance Ca(OH)2 is considered
A)a weak Arrhenius
Q37: Which one of the following will give
Q38: The substance NH3 is considered
A)a weak acid.
B)a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents