Aqueous solutions of phosphoric acid and sodium nitrite are combined, and the following equilibrium is established. 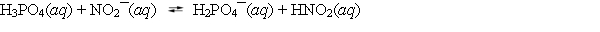 The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?
The equilibrium constant Kc for this reaction is greater than one. Based on this information, which of the following statements is correct?
A) Phosphoric acid is a weaker acid than nitrous acid.
B) Nitrous acid is a weaker acid than water.
C) The nitrite anion is a weaker base than the dihydrogen phosphate anion.
D) The dihydrogen phosphate anion is a stronger acid than nitrous acid.
E) Phosphoric acid is a stronger acid than nitrous acid.
Correct Answer:
Verified
Q46: A 0.15 M solution of chloroacetic acid
Q47: Formic acid, which is a component of
Q48: Select the pair of substances in which
Q49: Phosphoric acid, H3PO4, is a triprotic acid,
Q50: Lactic acid has a pKa of 3.08.
Q52: Arsenic acid, H3AsO4, is used industrially to
Q53: Butyric acid is responsible for the odor
Q54: A 0.050 M solution of the weak
Q55: A student adds 0.1 mol of oxalic
Q56: Farmers who raise cotton once used arsenic
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents