Write the mass-action expression, Qc, for the following chemical reaction. 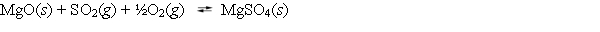
A) 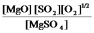
B) 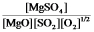
C) 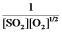
D) 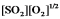
E) None of these choices is correct.
Correct Answer:
Verified
Q21: In water, the following equilibrium exists:
Q22: Write the mass-action expression, Qc, for the
Q23: What is the mass-action expression, Qc, for
Q24: The equilibrium constant for reaction (1) below
Q25: The equilibrium constant for the reaction of
Q27: Given this equilibrium constant data at
Q28: Consider the equilibrium reaction: Q29: At 500 Q30: The equilibrium constant, Kp, has a value Q31: Consider the equilibrium reaction: ![]()
![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents