Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. 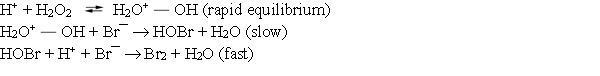 What is the overall reaction equation for this process?
What is the overall reaction equation for this process?
A) 2H2O+  OH + 2Br¯ H2O2 + Br2 + 2H2O
OH + 2Br¯ H2O2 + Br2 + 2H2O
B) 2H+ + 2Br¯ + H2O2 Br2 + 2H2O
C) 2H+ + H2O2 + Br¯ + HOBr H2O+  OH + Br2 + H2O
OH + Br2 + H2O
D) H2O+  OH + Br¯ + H+ Br2 + H2O
OH + Br¯ + H+ Br2 + H2O
E) None of these choices is correct.
Correct Answer:
Verified
Q44: Which of the following affects the activation
Q49: The kinetics of the decomposition of
Q50: In going from room temperature (25.0
Q51: Consider the following mechanism for the oxidation
Q52: The decomposition of dinitrogen pentaoxide to
Q55: A reaction has an activation energy
Q57: In the gas phase at 500.
Q59: The decomposition of dinitrogen pentaoxide has
Q66: An increase in temperature increases the reaction
Q76: In an exothermic reaction,
A)the forward reaction is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents