In the collision theory of reaction rates, the rate constant for a bimolecular reaction can be written as k = z · p · exp 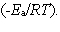 In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
A) z
B) p
C) exp 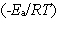
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: The half-life of a second-order reaction does
Q19: The units of the rate of reaction
Q63: The greater the energy of activation, Ea,
Q67: The elementary reaction HBr(g) + Br(g)
Q68: The gas-phase conversion of 1,3-butadiene to
Q71: You are studying the rate of
Q73: Is a bimolecular reaction necessarily second-order? Is
Q74: In the gas phase at 500.
Q75: You are required to determine the energy
Q75: A chemical reaction of the general
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents