Consider the general gas-phase reaction of a molecular substance, A:
1. 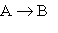 At very low pressures many such reactions occur by the following mechanism:
At very low pressures many such reactions occur by the following mechanism:
2. 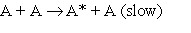 3.
3. 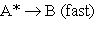 (A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
(A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
A) Which of the three reactions above is/are elementary?
B) Where appropriate, identify the molecularity of the reactions.
C) Show that the proposed mechanism is consistent with reaction 1, the observed reaction.
D) Given the mechanism above, suggest a likely rate law for reaction (1).
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: All bimolecular reactions are second-order reactions.
Q4: All second-order reactions are bimolecular reactions.
Q5: The half-life of a first-order reaction does
Q6: A reaction intermediate is a species corresponding
Q19: The units of the rate of reaction
Q63: The greater the energy of activation, Ea,
Q69: According to the collision theory of reaction
Q74: In the gas phase at 500.
Q75: A chemical reaction of the general
Q75: You are required to determine the energy
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents