Select the correct Lewis structure for nitrogen trifluoride, NF3.
A) 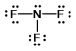
B) 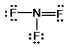
C) 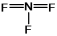
D) 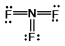
E) 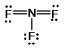
Correct Answer:
Verified
Q8: In which one of the following is
Q10: Hydrazine, N2H4, is a good reducing agent
Q11: Select the best Lewis structure for P2I4.
A)
Q15: In neutral molecules, how many bonds are
Q16: Which one of the following Lewis structures
Q17: Thionyl chloride is used as an oxidizing
Q21: In the nitrate ion (NO3−), nitrogen and
Q28: How many resonance structures are possible for
Q29: In carbon disulfide, how many lone pairs
Q40: How many electron pairs are shared between
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents