Oxygen difluoride is a powerful oxidizing and fluorinating agent. Select its Lewis structure.
A) 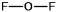
B) 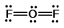
C) 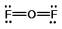
D) 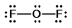
E) None of these choices is correct.
Correct Answer:
Verified
Q6: Select the best Lewis structure for ClCN.
A)
Q7: Select the correct Lewis structure for TeBr2.
A)
Q8: In which one of the following is
Q10: Hydrazine, N2H4, is a good reducing agent
Q11: Select the best Lewis structure for P2I4.
A)
Q12: In which one of the following is
Q29: In which one of the following species
Q29: In carbon disulfide, how many lone pairs
Q34: Which one of the following molecules contains
Q38: In which one of the following molecules
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents