In which one of the following structures does the central atom have a formal charge of +2?
A) SF6 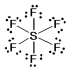
B) SO42¯ 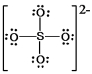
C) O3 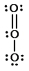
D) BeCl2 
E) AlCl4¯ 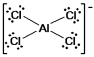
Correct Answer:
Verified
Q15: In neutral molecules, how many bonds are
Q16: Which one of the following Lewis structures
Q17: Thionyl chloride is used as an oxidizing
Q19: Select the correct Lewis structure for NOCl,
Q20: Which one of the following Lewis structures
Q25: Phosphoryl iodide is used in the preparation
Q26: Considering all the bonds in a
Q50: According to VSEPR theory, a molecule with
Q51: How many faces and how many vertexes
Q52: Which of the following atoms can expand
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents