Gallium has two naturally occurring isotopes with the following masses and natural abundances.Calculate the average atomic mass of Ga. 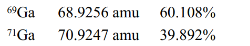
A) 69.925 amu
B) 70.127 amu
C) 70.000 amu
D) 69.824 amu
E) 69.723 amu
Correct Answer:
Verified
Q67: You synthesize a superheavy atom that fits
Q68: What ion would you predict element 118
Q69: How many CaCl2 formula units are in
Q70: You create a superheavy atom with an
Q71: What is the formula unit mass of
Q73: Identify the element based on the following
Q74: Identify the element based on the following
Q75: Which has the highest molecular mass?
A)Br2O
B)IBr2
C)CBr4
D)Br2O8
E)BrF5
Q76: One isotope makes up 97% of all
Q77: For each of the elements below, there
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents