Which of the transitions in the hydrogen atom energy-level diagram shown here is not possible? 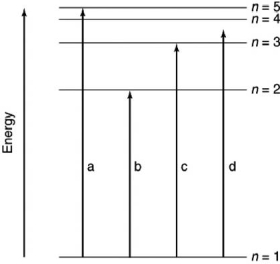
A) a
B) b
C) c
D) d
E) all are possible
Correct Answer:
Verified
Q54: Which statement below regarding the Bohr model
Q55: The energy change for an electronic
Q56: The absorption and emission spectra of an
Q57: Work function values are often given in
Q58: Determine the wavelength of the line in
Q60: How many times longer is the
Q61: What is the approximate total uncertainty
Q63: Which of the following objects, all moving
Q64: What is the de Broglie wavelength of
Q66: De Broglie reasoned that for the electron
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents