Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 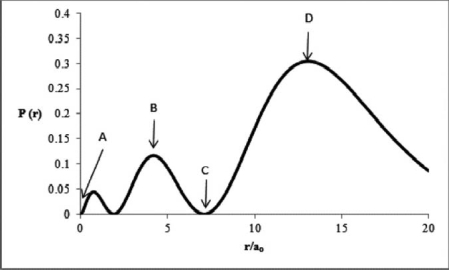 Identify:
Identify:
1) where the atomic nucleus is located,
2) a radial node,
3) the most probable distance of the electron from the nucleus, and
4) the principal quantum number for this orbital.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q151: What is meant when two or more
Q166: Write the electron configuration of Pd.
Q166: Identify which element, potassium or bromine, has
Q167: Arrange the following elements in order of
Q168: Copper commonly forms a cation with a
Q169: Arrange the following elements in order of
Q170: Write the electron configuration of Ru3+.
Q171: How many electrons can be in the
Q173: Write the electron configuration of Cr.
Q175: Write the condensed electron configuration of Po.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents