Which statement below regarding the Lewis structure of trichloroacetic acid, CH 2Cl3O2 is FALSE? A partial bonding framework is given. 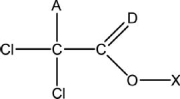
A) The atom at position "X" is hydrogen.
B) In the correct and complete Lewis structure, the atom at position "D" violates the octet rule.
C) The atom at position "A" is Chlorine.
D) The oxygen atom shown has two pairs of nonbonding electrons.
E) In the correct and complete Lewis structure, all of the chlorine atoms have three pairs of nonbonding electrons.
Correct Answer:
Verified
Q76: How many shared electrons are there in
Q77: Which molecule below contains a double bond?
A)N2
B)CO
C)S2
D)CCl4
E)Cl2
Q78: Which is the correct Lewis symbol for
Q79: How many shared electrons are there in
Q80: How many shared electron pairs are there
Q82: Based on its Lewis dot structure and
Q83: Nitrite (NO2-) is an important nutrient in
Q84: What type of bonds are the external
Q85: Which chemical species below has the most
Q86: How many valence electrons are contained in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents