Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below.Explain why you think this molecule is planar or nonplanar. 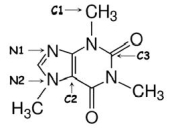
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q126: Carbonyl dihalides (COX2 with X = I,
Q127: Draw the molecular orbital diagram for HCl.Use
Q128: Draw the Lewis structure of Cl2O.Give the
Q129: Identify the hybridization of atomic orbitals for
Q130: Explain the phenomenon of light absorption and
Q132: A Lewis structure of aspirin without the
Q133: Give the molecular orbital valence electron configuration
Q134: Use the 1s orbital of hydrogen
Q135: Draw the Lewis structure of BrF5.Give the
Q136: Answer the following questions about the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents