The relative energies (strengths) of the intermolecular forces present in each of five different pure substances are shown in the figure below.Which substance has the lowest melting point? 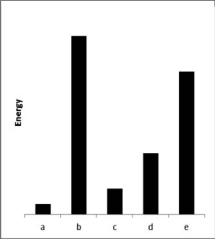
A) a
B) b
C) c
D) d
E) e
Correct Answer:
Verified
Q1: Ion-dipole forces always require_
A)an ion and a
Q3: Which molecule below exhibits the weakest dispersion
Q4: Which compound below has the highest boiling
Q5: Arrange the following compounds in order
Q6: Which statement below regarding London dispersion forces
Q9: Which molecule below has the highest polarizability?
A)N2
B)O2
C)F2
D)Br2
E)I2
Q10: The relative energies (strengths) of the intermolecular
Q11: The relative energies (strengths) of the intermolecular
Q12: Which type of intermolecular interaction exists for
Q21: Polarizability refers to _
A)the ease with which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents