Which statement below about the following phase diagram is FALSE? 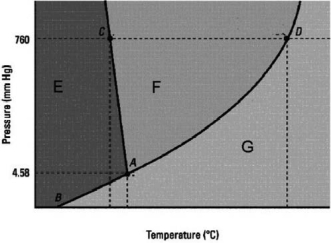
A) The solid phase is more dense than the liquid phase.
B) D is the normal boiling point.
C) The melting point decreases as pressure increases.
D) The normal melting point is at a lower temperature than the triple point.
E) E, F, and G label in that order the solid, liquid, and gas phases.
Correct Answer:
Verified
Q61: Consider the phase diagram for a substance
Q62: Which statement below best explains why
Q63: The temperature at point b in the
Q64: Arrange the following molecules in order of
Q65: Point c in the phase diagram below
Q67: Which statement below regarding capillary action is
Q68: Difluoromethane (CH2F2) has a dipole moment
Q69: Which liquid below will have the highest
Q70: The temperature at point a in the
Q118: Water forms a concave meniscus in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents