The reaction of sulfur dioxide gas with oxygen to form sulfur trioxide gas at 25 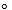 C and 1 atm releases approximately 198.5 kJ of heat while absorbing approximately 2.5 kJ of work.Which of the following is true?
C and 1 atm releases approximately 198.5 kJ of heat while absorbing approximately 2.5 kJ of work.Which of the following is true?
2SO2 (g) + O2(g) 2 SO3(g)
A) The reaction enthalpy cannot be determined without more information.
B) The reaction is endothermic and E is less positive than H.
C) The reaction is endothermic and E is more positive than H .
D) The reaction is exothermic and E is more negative than H .
E) The reaction is exothermic and E is less negative than H .
Correct Answer:
Verified
Q50: Sodium azide (NaN3) decomposes to form sodium
Q51: Enthalpy change is defined as
A)the energy that
Q52: The reaction of nitrogen with oxygen
Q53: In a steam engine, steam in a
Q54: In a steam engine, steam in a
Q56: The volume change associated with a
Q57: Which of the following reactions would
Q58: In a steam engine, steam in a
Q59: A cup of coffee is heated
Q60: Water has a molar heat capacity
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents