When trinitrotoluene TNT) detonates according to the reaction
2 C7H5N3O6 (s) 3 N2(g) +5 H2(g) + 12 CO(g) + 2 C(s) , the enthalpy change at 25 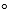 C and 1 atm is -1475 kJ while the volume increases by about 489 L.What is the internal energy change (assume only P-V work is done) ?
C and 1 atm is -1475 kJ while the volume increases by about 489 L.What is the internal energy change (assume only P-V work is done) ?
A) +986 kJ
B) (-1525 kJ)
C) (-1425 kJ)
D) +1425 kJ
E) (-1964 kJ)
Correct Answer:
Verified
Q44: When one mole of zinc and
Q45: How much energy is needed to
Q46: Which statement below regarding heat capacity
Q47: Which statement below regarding heating curves is
Q49: The heating curve for a substance is
Q50: Sodium azide (NaN3) decomposes to form sodium
Q51: Enthalpy change is defined as
A)the energy that
Q52: The reaction of nitrogen with oxygen
Q53: In a steam engine, steam in a
Q57: During an exothermic process, _ for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents