Coal is converted into cleaner, more transportable fuels by burning it with oxygen to produce carbon monoxide.The carbon monoxide then is reacted with hydrogen using a catalyst to produce methane and water.Is the reaction between CO and H2 exothermic or endothermic, and what is the change in enthalpy for it? The enthalpies of formation of the reactants and products are given below. 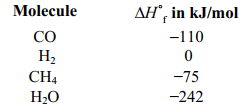
A) exothermic, +317 kJ/mol
B) endothermic, +207 kJ/mol
C) exothermic, - 427 kJ/mol
D) endothermic, +427 kJ/mol
E) exothermic, -207 kJ/mol
Correct Answer:
Verified
Q6: Coulomb's law states that the interaction energy
Q51: Which of the following is needed to
Q122: Which of the ionic compounds below would
Q123: Determine the energy change for the
Q124: Which statement below regarding the dissolution of
Q127: Based on lattice energies, which listing
Q128: Which of the following requires the smallest
Q129: Which ionic compound below would you expect
Q130: Which of the following compounds has the
Q131: Which ionic compound below would you expect
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents