Use the following data to calculate the enthalpy change for the following reaction: 2 Na(s) + 1/2 O2 (g) Na2 O(s) 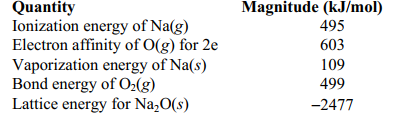
A) (-1322 kJ/mol)
B) (-1571 kJ/mol)
C) (-417 kJ/mol)
D) (-771 kJ/mol)
E) (-557 kJ/mol)
Correct Answer:
Verified
Q130: Which of the following compounds has the
Q131: Which ionic compound below would you expect
Q132: Which statement below regarding the solubility of
Q133: Which statement below regarding the solubility of
Q134: Based on lattice energies, which of
Q136: Which of the following has the largest
Q137: Which of the following processes is
Q138: Which of the following will require the
Q139: Which ionic compound below would you expect
Q140: Which of the following is NOT typically
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents