Use the following data to calculate the enthalpy change for the following reaction.
2 Na(s) +O2(g) Na2O(s) 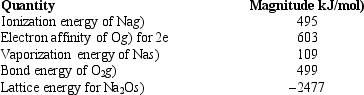
Correct Answer:
Verified
Q127: Rank the following ionic compounds in order
Q137: C8H18 (114 g/mol, d = 0.69 g/mL)
Q205: A new diet fad has just
Q206: Briefly explain how bond energies can be
Q207: The basal metabolic rate (BMR) is the
Q208: Calculate the enthalpy of reaction for the
Q209: Complete the table below, and identify the
Q211: The basal metabolic rate (BMR) is the
Q213: Complete the table below, and identify the
Q214: Gunpowder is usually a mixture of potassium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents