Calculate the root-mean-square speed of an O2 molecule with an average kinetic energy of 6.2 * 10-21 J at 25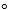 C.
C.
(1 J = 1 kg . m2/s2; 1 mol = 6.02 * 1023)
A) 20 pm/s
B) 230 km/s
C) 480 m/s
D) 120 m/s
E) 230 m/s
Correct Answer:
Verified
Q25: The diffusion rate of helium in
Q26: What is the root-mean-square speed of
Q27: Determine the root-mean-square speed of methane (CH4)
Q28: At a given temperature, the effusion rate
Q29: Helium-neon (HeNe) lasers contain a low-pressure mixture
Q31: Which set of gases is listed
Q32: Air is primarily nitrogen and oxygen, but
Q33: At a given temperature, the rate at
Q34: Calculate the average kinetic energy of an
Q35: Calculate the average kinetic energy of CO2
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents