The van der Waals constants for NO2 are a = 5.28 L2 .atm/mol2 and b = 0.04424 L/mol.What is the pressure of 56.33 g of NO2 in a 15.00 L container at 22.00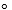 C when it is treated as
C when it is treated as
A) ideal and
B) real?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q147: A scuba diver releases a balloon containing
Q154: One of the highest temperatures ever
Q155: What pressure (in Pa) will be
Q156: Define mean free path.How does the mean
Q157: How many moles of an ideal
Q158: A gas has a density of
Q159: In an experiment, 2.000 grams of
Q161: He and O2 gases are mixed at
Q163: Three bulbs are connected as shown in
Q164: Explain why the constants a and b
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents