Which statement regarding the boiling of a mixture of liquids A and B is NOT correct? 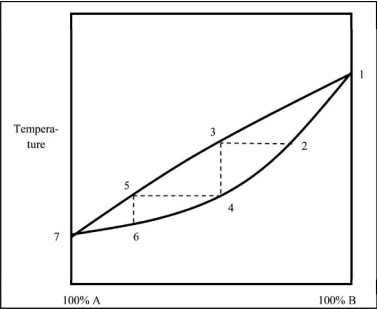
A) The distillate collected boils at lower temperatures as the percentage of A increases.
B) The distillate always contains a higher percentage of B because pure B is less volatile than pure A.
C) The upper curve reflects the composition of the vapor at a given temperature.
D) As the distillation progresses from point 2 to point 6, the liquid becomes richer in A.
E) The boiling point of pure A is lower than that of pure B.
Correct Answer:
Verified
Q43: Which statement regarding nonideal solutions is NOT
Q44: A solution is prepared by mixing
Q45: The smell of fresh-cut pine is due
Q46: Thiophene, C4H4S, is a fairly volatile
Q48: A solution is prepared by mixing
Q49: Gasoline is primarily a mixture of
Q50: The aroma from almonds and cherries is
Q51: A solution is prepared by mixing
Q52: A solution is prepared by mixing
Q68: What physical property is used to separate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents