What is the overall standard free energy change for the following two reactions in terms of G1 and G2?
1) A + B C G 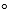 rxn = G1
rxn = G1
2) 2 C + D E G 11eed8bf_d792_f6ef_a0ba_3befc13c5334_TB6562_11 rxn = G2
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q121: Determine the normal melting point of
Q122: Estimate the free energy change for the
Q123: The standard molar enthalpy of fusion
Q125: Suppose a fermentation process occurs in
Q127: Phosphine can theoretically be made by
Q128: Determine the change in the standard
Q129: The change in standard molar entropy,
Q130: Determine the standard free energy change
Q165: What are the signs of
Q186: What is a microstate and how are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents