The rate of disappearance of HI in the reaction 2 HI(g) I2 (g) +H2 (g) is shown in the following figure.Estimate the initial rate of reaction. 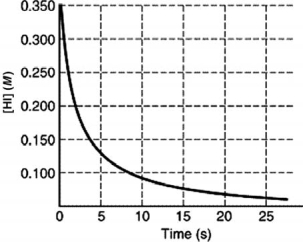
A) 0.350 M/s
B) 0.140 M/s
C) 0.035 M/s
D) 0.175 M/s
E) 0.070 M/s
Correct Answer:
Verified
Q39: In a rate law, the partial orders
Q40: Given the following data, determine the
Q41: The reaction C2 H5 Cl(g)
Q42: The second-order reaction A
Q43: Determine the overall order of the
Q45: The half-life (t1/2) of a first-order reaction
Q46: Determine the overall order of the
Q47: Given the following data, determine the
Q48: Suppose 2.00 g of azomethane (58.08
Q49: For the rate law Rate =k[A]3/2[B],
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents