Determine the overall order of the reaction H2 (g) + 2 ICl(g) I2 (g) 2 HCl(g) from the following data: 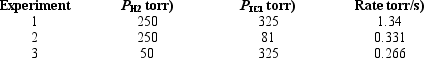
A) first
B) second
C) third
D) fourth
E) zero
Correct Answer:
Verified
Q49: For the rate law Rate =k[A]3/2[B],
Q50: For the rate law Rate =k[A]1/2[B],
Q51: Which statement below regarding rate laws and
Q52: Which of these could be the units
Q53: A reaction is first order in A.If
Q55: Which of these could be the units
Q56: Given the following data, determine the
Q57: The second-order reaction A
Q58: A second-order reaction (2 A
Q59: The reaction A + 2B
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents