Given the following data, determine the rate constant of the reaction 2 NO(g) + CL2 (g) 2 NOCl(g) .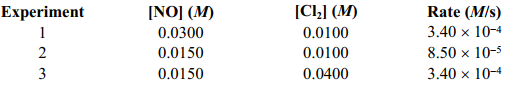
A) 1.13 M -2s-1
B) 9.44 M -2s-1
C) 37.8 M -2s-1
D) 0.0265 M -2s-1
E) 59.6 M -2s-1
Correct Answer:
Verified
Q70: Given the following data, determine the
Q71: The half-life of radioactive carbon-14 is 5730
Q72: The rate constant for the decomposition reaction
Q73: The first-order reaction A
Q74: Dinitrogen pentoxide rapidly decomposes in the
Q76: Dinitrogen pentoxide rapidly decomposes in the
Q77: The rate constant for the reaction
Q78: Nitrogen dioxide undergoes thermal decomposition according
Q79: The half-life for the second-order decomposition reaction
Q80: Given the following data, determine the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents