The following energy profiles for four different reactions are shown.Which reaction is the most exothermic? 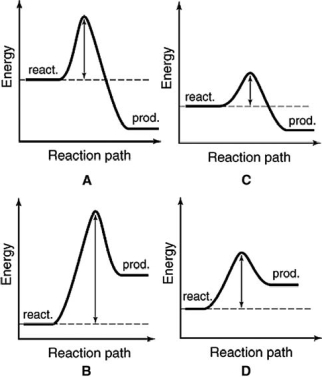
A) A
B) B
C) C
D) D
E) More information is required.
Correct Answer:
Verified
Q79: The linear form of _ is very
Q92: The following figure shows Arrhenius plots for
Q93: The linear form of the Arrhenius equation
Q94: Which statement below regarding kinetic molecular theory
Q96: The energy profiles for four different reactions
Q98: Which statement below regarding transition states and
Q99: Which statement below regarding collision energy and
Q100: The following figure shows Arrhenius plots for
Q102: Which of the following describes a reaction
Q113: A reaction mechanism consists of a series
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents