The observed rate law for the reaction H2 (g) + 2 ICl(g) I 2 (g) + 2 HCl(g) is first order with respect to each reactant.Draw an energy diagram that shows energy versus reaction progress for the mechanism given.Both steps are exothermic.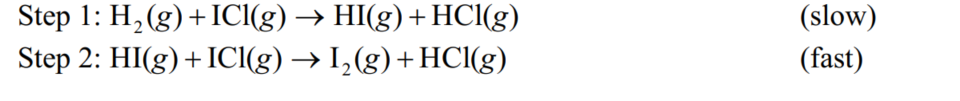
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q156: The reaction NO2 (g) +CO(g)
Q157: For the reaction
Q158: Chlorine atoms react with methane, CH4
Q160: For the second-order reaction
Q160: In an elementary step of a reaction
Q162: In an _ reaction, the activation
Q163: The experimental rate law for the photochemical
Q164: The observed rate law for the
Q165: The rate at which gas-phase hydrogen
Q166: Write rate laws for the following elementary
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents