Given the following Ka values, which of the following acids is the strongest in water? 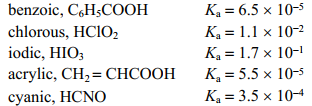
A) benzoic
B) chlorous
C) iodic
D) acrylic
E) cyanic
Correct Answer:
Verified
Q2: Which of these is a strong acid
Q8: Which of the following statements regarding acid
Q9: Which of the following is a strong
Q10: Given the following Ka values, which of
Q12: Which of the following is a strong
Q12: Which statement about nitrous acid and nitric
Q14: Use the following acid ionization constants
Q15: The degree of ionization of a strong
Q16: Which of the following is NOT a
Q17: The degree of ionization of a weak
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents