Using the following data, determine Eocell for the electrochemical cell constructed using the reaction of nitrous oxide and oxalic acid under acidic conditions.Unbalanced: N2O(g) + H2C2O4( s) 2 CO2 (g) +N2 (g) +H2 O(l) 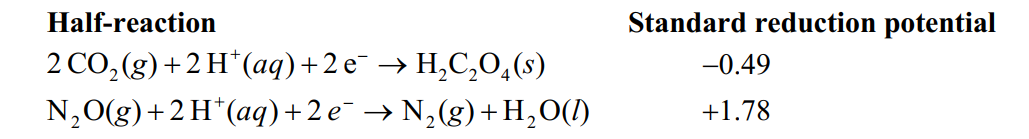
A) (+1.29 V)
B) (-1.29 V)
C) (+2.27 V)
D) (-2.27 V)
E) (+0.87 V)
Correct Answer:
Verified
Q6: Oxidation is the _
A)gain of electrons.
B)loss of
Q16: Which statement below about a cathode in
Q17: Glancing at a periodic table, where do
Q19: What is the oxidation number of sulfur
Q20: Consider the reaction, Sn + Au3
Q22: If the potential of a voltaic cell
Q23: Which statement below regarding reduction potentials is
Q24: Which one of the following items does
Q26: Using the following data, determine the
Q66: The work involved in moving exactly 1
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents