Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has iron Fe) and magnesium Mg) electrodes immersed in 1M Fe3+and Mg2+solutions? Also, identify the cathode. 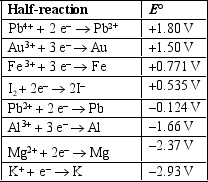
A) (+1.60 V with Fe as the cathode)
B) (-1.60 V with Mg as the cathode)
C) (-3.14 V with Fe as the cathode)
D) (-3.14 V with Mg as the cathode)
E) (+3.14 V with Fe as the cathode)
Correct Answer:
Verified
Q34: The magnitude of the charge on a
Q35: Silver tarnish( Ag2S) can be removed by
Q36: In one episode of the 1960s television
Q37: The spontaneous redox reaction in a
Q38: Silver tarnish (Ag2S) can be removed
Q40: Identify the strongest reducing agent in the
Q41: An electronic device requires two 1.50-V AA
Q42: What is the cell potential for
Q43: What is true when a battery voltaic
Q44: The standard hydrogen electrode is
A)used to calibrate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents