Calculate G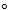 for an electrochemical cell reaction that occurs under basic aqueous conditions based on the following two half-reactions for which the standard reduction potentials are given.Use the smallest whole-number coefficients possible when balancing the overall reaction.
for an electrochemical cell reaction that occurs under basic aqueous conditions based on the following two half-reactions for which the standard reduction potentials are given.Use the smallest whole-number coefficients possible when balancing the overall reaction.
Cd(OH) 2 + 2e- Cd + 2 OH- -0.824 V
NiO(OH) + H2 O +e- Ni(OH) 2 +OH- +1.32 V
A) (-669 kJ)
B) (+95.7 kJ)
C) (-95.7 kJ)
D) (-414 kJ)
E) (+414 kJ)
Correct Answer:
Verified
Q46: The Nernst equation can be used to
Q47: An electrochemical cell is constructed with a
Q48: An electrochemical cell contains a standard hydrogen
Q49: An electronic device requires four 1.50-V AA
Q50: Neuron cells generate electrical signals by concentration
Q52: A concentration cell is constructed by using
Q53: The change in free energy for
Q54: An electrochemical cell is constructed with a
Q55: Which statement does NOT correctly describe a
Q56: Which statement does NOT correctly describe
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents