For the electrochemical cell based on the reaction of zinc metal with copperII) ions indicated below where zinc is being oxidized, identify the components labeled as a-e.The anode is on the left; the cathode is on the right.Use the terms zinc nitrate solution, copper nitrate solution, copper electrode, zinc electrode, and salt bridge.Also, label the electrodes as+ and -. 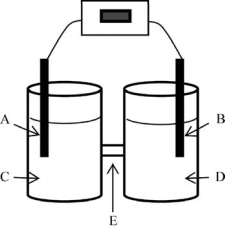
Correct Answer:
Verified
B) copper...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q112: Methanol fuel cells depend on the
Q113: The oxidation of hydrogen by oxygen
Q114: Silver tarnishes due to the formation of
Q115: When a reduction half-reaction is written as
Q116: The following reaction is called the
Q119: The oxidation of methanol, as described
Q120: What must be true about the
Q122: A single cell in a lead acid
Q124: For a chemical reaction to be considered
Q165: The capacity of a battery usually
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents