In an experiment, 100 mL of a 0.1 M solution of methionine is titrated using 0.1 M NaOH.Using this information, along with the titration curve shown, which of the following statements is NOT correct? 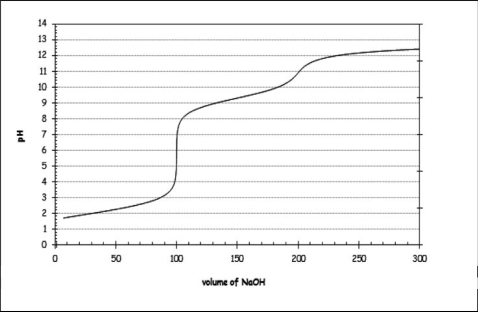
A) At low pHs, the majority of the amino acid present is fully protonated.
B) The pKa of the protonated amine group seems to be a little greater than 10.
C) When the pH reaches the physiological value of 7.4, the carboxylic acid group is essentially deprotonated.
D) The Ka of the -COOH group appears to be between 5 *10-3 and 8*10-3.
E) Methionine appears to have a neutral side group, as there seem to be two equivalence points evident on the titration curve.
Correct Answer:
Verified
Q6: Aspartame exists as a zwitterion at physiological
Q7: Which statement about amino acid enantiomers is
Q8: Which of the following functional groups is
Q9: Isomers that cannot be superimposed on their
Q10: A peptide bond is the result of
Q12: An organic molecule present naturally in a
Q12: At a physiological pH of 7.4, amino
Q13: Amino acids are bonded together via_ bonds.
A)glycosidic
B)peptide
C)hydrogen
D)ionic
E)van
Q14: At a physiological pH of 7.4, amino
Q16: Amino acids that humans cannot synthesize are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents